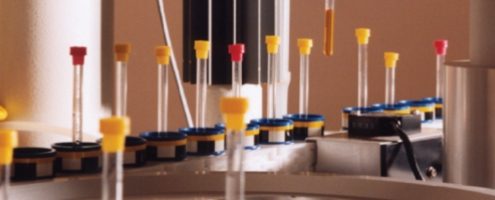
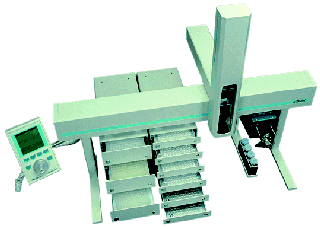
Protasis hosted a table at this year’s Small Molecule NMR (SMASH) Conference on September 9-12 in Providence, RI. We set up a One-Minute NMR system and ran it through its sample loading paces. The conference was held jointly with CoSMoS, the Society for Small Molecule Science with about 300 total attendees. Protasis showed two joint posters with our customer collaborators on CryoFlow NMR:
Poster #49
Improvements in Flow-Injection NMR as a Tool for High-Throughput Sample Analysis
Paul Krolikowski (1), L. Steven Hollis(1), Roger Kautz (2) and David Strand (3)
1. Molecular Structure, Amgen Inc., Cambridge, MA, USA
2. Barnett Institute at Northeastern University, Boston, MA, USA
3. Protasis Corporation, Marlboro, MA, USA
Recently, dramatic improvements in S/N have been obtained using customized flow-NMR instrumentation by incorporating a small volume flow-cell (10-60 uL) with a high-sensitivity 600 MHz Bruker 5-mm TCI-cryoprobe and a Protasis sample delivery system. The large sensitivity gain provided by cryoprobe technology (as much as 20X increase compared to a micro-coil 500 MHz RT flow-probe) has dramatically reduced data collection times to a point where routine 1H and g-HSQCdept spectra can be collected on small samples (25 uL at 20 mM) in under 10 minutes. These hardware improvements have allowed implementation of rapid quantitative-NMR analysis for HTPP (High Throughput Purification Process) samples using Digital-Eretic referencing as an integral calibration technique. In addition, new flow-cell inserts have been designed which easily can be interchanged to alter sample volume or switched from flow to tube based operations in just a few minutes with minimal setup time.
The concept of tailoring the flow-cell volume to match that of the intended sample stream (with an active volume of 10, 30 or 60 uL) using a standard Bruker pass-through cryoprobe, will be discussed as part of our continuing effort to improve NMR sampling efficiency. The Protasis sample delivery station and operating system (One-Minute NMR) provides a flexible and convenient platform for submitting samples in 96-well plate format, or as single samples in walk-up mode. Automated data processing using ACD Automation Server provides a reliable solution for archiving and distributing the large amounts of processed data that are generated from plate-base HTPP submissions. In addition, segmented-flow NMR methods (SFA), using a susceptibility matched push-solvent (FC-43) to limit sample dispersion in the flow cell, are also being investigated in an effort to improve S/N with mass-limited samples. The initial results of these studies, which show up to a two-fold improvement in S/N vs. single-solvent push techniques, will be presented.
Poster #21
Segmented Flow Into LC Probes Double Flow-NMR Sensitivity and Throughput
Roger Kautz (1), David Strand (2), and Steve Hollis (3)
1. Barnett Institute at Northeastern University, Boston, MA
2. Protasis Corporation, Marlboro, MA
3. Amgen Corporation, Cambridge, MA
* Maintains uniform original concentration, for ligand-binding or qNMR studies.
* Confines sample in NMR coil volume, for optimal sensitivity.
* Improves throughput by 10 min/sample; reduces solvent consumption 5-fold.
* Applicable to cryo-flow for trace sensitivity, or old LC probe for high-throughput.
Flow-injection NMR (FIA-NMR) has proven valuable for acquiring spectra directly from vials or 96-well plates, avoiding the time and expense of preparing samples in tubes, and specialized automation to load tubes. Extending our previous work in segmented flow loading of microcoil probes (“microdroplet NMR”) [1], we here show how segmented flow (SFA) loading solves several problems seen with conventional flow-NMR into LC probes (60 uL or larger).
Segmented flow loads samples without dispersion or dilution — the sample is transported like a drop of water in oil. The flow system is filled with an immiscible fluid (fluorocarbon FC43) so the sample moves as a droplet or, in narrow tubing, as an elongated plug with a sharp boundary and with uniform (undiluted) concentration throughout. FC43 has magnetic susceptibility matched to D2O, and functions as “Shigemi tubes for a flow system” to confine the entire sample within the observed volume. To implement segmented flow, use of a fluorinated fluid in Teflon tubing is ideal; fluoro-silanized glass surfaces also work well. The data presented were obtained by using a stock commercial flow-NMR platform (Protasis One-Minute NMR) with a Teflon sample loop and transfer line. SFA and FIA were compared in in video of injected dye, and in on-flow and stopped-flow NMR spectra of a standard (20 mg/mL caffeine in D2O).
Results: SFA loading was explored in a cryo-flow system with a 60 uL nominal NMR observed volume (120 uL flowcell volume; 150 uL dead vol to center of V-obs). SFA loading 70 uL of the standard gave twice (2x) the NMR signal intensity as FIA loading; which was 95% the signal intensity of a flowcell filled with the standard. A 50 uL sample could be shimmed to the same lineshape as 70 uL or larger, but accordingly shows only 70% of the signal strength (50 uL/70 uL = 71%). Video of dye injections by SFA and FIA provide a concrete picture. Samples loaded with SFA move into the detected volume as a discrete object, like a car into a garage; accordingly, on-flow NMR signal strength rises linearly from first light to 100% as the leading edge of sample fills the coil volume; plateaus at 100% while the coil is filled, then linearly decreases to 0 as the trailing boundary of the sample passes out of the detector region. FIA samples, in contrast, arrive like the hot water at a distant tap: signal intensity increases more slowly with a slope projecting to 100% after 0.7 dead volumes, but cresting and tailing for smaller samples. Parabolic flow in the flowcell produced large concentration gradients both radially and axially, reflected in the lineshape (30 Hz) of the caffeine methyl resonances, which are concentration-sensitive. When flushing samples, the tailing in FIA required 300 uL (nearly 3 flowcell volumes) to bring residual signal below 2%.
Applications: Loading samples at a defined and uniform concentration enables ligand-binding or qNMR studies. Confining samples into the observed volume gives the theoretical optimum sensitivity for a probe, under unattended automation. SFA-NMR with cryoprobes gives high sensitivity; an old LC probe would serve for high throughput of routine chemistry samples.
1. Lin et al, Anal. Chem 80: 8045 (2008)
SMASH 2012 program: http://www.smashnmr.org/images/previous_smash/SMASH2012/smashProgram.pdf